
From the table above, a single bond between fluorine and nitrogen has a bond length of approximately 64 + 71 =135 pm. The bond between fluorine and nitrogen is a single bond. To find the nitrogen-to-fluorine bond length in NF 3, draw the Lewis structure. Therefore, the bond length is greater in CO 2.ĥ. Therefore, the bond length is greater in CO 2.Īnother method makes use of the fact that the more electron bonds between the atoms, the tighter the electrons are pulling the atoms together. Referring to the table above, a double bond between carbon and oxygen has a bond length of approximately 67 + 57 = 124 pm and a triple bond between carbon and oxygen has a bond length of approximately 60 + 53 =113 pm. From the Lewis structures for CO 2 and CO, there is a double bond between the carbon and oxygen in CO 2 and a triple bond between the carbon and oxygen in CO. There is a formation of two single bonds and one double bond between three atoms.

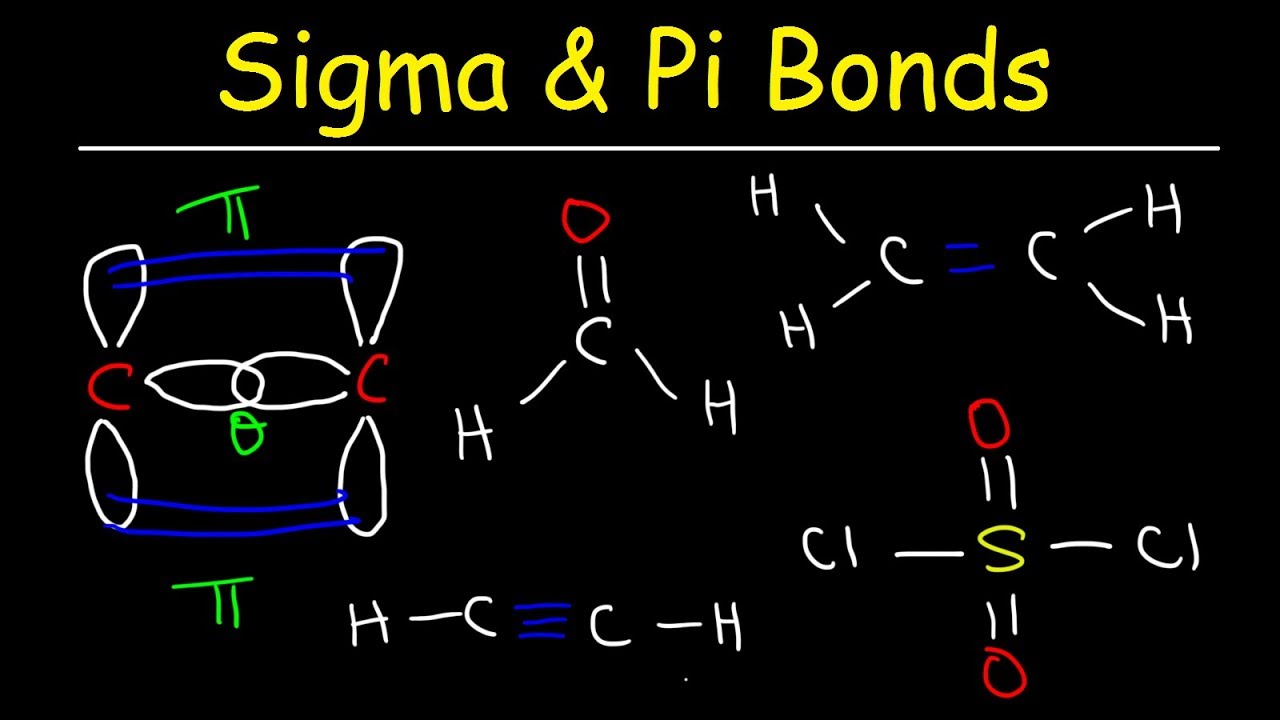
A carbon atom is sp2 hybridized when bonding takes place between 1 s-orbital with two p orbitals. The bond between carbon and nitrogen is a triple bond, and a triple bond between carbon and nitrogen has a bond length of approximately 60 + 54 =114 pm.Ĥ. When the hybridization occurs the molecules have a linear arrangement of the atoms with a bond angle of 180°. To find the carbon-nitrogen bond length in HCN, draw the Lewis structure of HCN. Adding these together and dividing by the number of bonds (3) reveals that the bond order of nitrate is 1.33.ģ. N=O has a bond order of two, and both N-O bonds have a bond order of one.
To find the bond order of this molecule, take the average of the bond orders. The Lewis structure for NO 3 - is given below: There is a double bond between the two oxygen atoms therefore, the bond order of the molecule is 2.Ģ.

First, write the Lewis structure for \(O_2\).


 0 kommentar(er)
0 kommentar(er)
